Login
Login
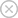
X-ray fluorescence spectrometer (XRF) is an analytical instrument used in the fields of steel, non-ferrous metals and precious metals, cement, petrochemicals, Geology, environment, biology, food, electronic materials, archeology, jewelry non-destructive testing and other fields.
XRF can analyze elements with a content of >0.0001% in massive solids, pressed or loose powders, fusion glass disc, liquids, metal disc, granular and film samples, The XRF instrument uses X-ray fluorescence to determine the elemental composition of the sample to conduct qualitative and quantitative analysis. The accuracy of the analysis relies heavily on the quality of the sample preparation, as any inconsistencies or contamination can lead to inaccurate results.
Massive metal samples can be analyzed directly after being cut or grinded. For non-metallic solid samples, Powder Pressed Disc and Fusion Glass Disc samples preparation methods are mainly used in XRF analytical field nowadays.
The method for pressing powder samples is: grind the sample to more than 200 mesh (grain size less than 0.074mm), use an aluminum box as the sample container, or use a polyester ring as a rim or a boric acid rim as a substrate. Directly pressed into discs under pressure. If the adhesion of the test itself is poor, you can also add a certain amount of binder to the test, grind it evenly, and then press it into tablets.
This method is simple and fast, but has large mineral effects, grain effect, matrix effect, poor sample consistency, and poor measurement precision. It is suitable for control analysis or qualitative and semi-quantitative analysis at the production site.
In the steel industry, mining industry, cement industry, precious metal industry, etc., a large number of samples are detected using traditional methods that cannot keep up with the production requirements. When performing X-ray fluorescence analysis, due to the complexity of the sample, the detection of X-ray fluorescence has mineral effect, matrix effect, grain effect and other issues hinder the accuracy of analysis. The fusion method basically solves the above problems, and the process is simple, which is of great significance for establishing the standard curve of X fluorescence.
There are three types of fusion furnaces: gas furnace, electric furnace and high-frequency induction fusion furnace. Duo to gas furnace and electric furnace show many disadvantages such as unequal temperature distribution, poor mixing, environmental problems, high operation costs and larger fusion, the high frequency Induction Fusion method is known as the highest degree accuracy sample preparing method in analytical field at present.
High-frequency Induction Fusion uses the induction principle to directly generate eddy currents in the crucible (it will not cause the temperature of the working environment to rise, there will be no feeling of baking, and there is no need to wear high-temperature work gloves).
The eddy currents guarantee that the melt has an optimal mix by the so-called bath movement. By that a constant and homogeneous quality of the melt is achieved. Accuracies of analysis and economy with optimal sample quality confirm the high quality of this method with a minimum of elemental losses, which can be rectified.
- Induction heating requires no warm-up or cool-down cycle, stand-by heat losses are reduced to a bare minimum
- Centrifugal variable speed mixing system improves the homogeneity and can also be used for high melting point materials. Fusion temperatures up to 1500℃ will be achieved.
- Rapid heating: from room temperature to 1000 degrees in 30 seconds
- High sample throughput as many as 100 samples more per day can be prepared with 8 hours operation.
- Easy operation: controlled by PLC with Touch panel user interface and 10 user-definable programs can be saved. Thereafter the working steps will run automatically.
- Separated fusion and molding design avoid rough bottom of Pt/Au crucible, solve the problem of polish, and extend service life of Pt/Au crucible. Therefore, cost will be lowered and efficiency also can be improved.
- Precise temperature controlling ensures quality of sample
- High quality of samples: homogeneity, no bubbles and smoothness
- minimum of elemental losses
- Eliminates the grain effect, matrix effect and mineral effect
- highest degree accuracy of analysis result
- Good for sample preparation of oxidic material for XRF analytical system.
- low energy consumption: only heat the crucible, heat the mold before casting
- environment friendly: Built-in exhaust system
- good working condition: reduced heat radiation
In summary, preparing samples for XRF spectrometer analysis is a meticulous process that requires attention to detail and adherence to strict protocols. By following proper sample preparation techniques, you can ensure accurate and reliable results from your XRF instrument. Remember to prioritize sample homogenization, accurate weighing and measurement, and proper handling to obtain the best results in XRF analysis.
In summary, preparing samples for XRF spectrometer analysis is a meticulous process that requires attention to detail and adherence to strict protocols. By following proper sample preparation techniques, you can ensure accurate and reliable results from your XRF instrument. Remember to prioritize sample homogenization, accurate weighing and measurement, and proper handling to obtain the best results in XRF analysis.
101 0 0
Previous: How Do Custom Bale Clamp Solutions Work?
Join Us
Comments
All Comments ( 0 )